Hypercalcaemia of Malignancy: A Foreboding Entity
Patients diagnosed with cancer often ask a reasonable question: “Why was my disease not picked up earlier?” The obvious explanation is that cancer of any type is an insidious disease entity that often only comes to medical attention when obstruction, direct invasion of critical structures — either by the primary or metastases — or metabolic mayhem ensues.
Biochemical changes associated with cancer can take many forms: unexplained anaemia or blood loss, derangement of liver function tests (LFTs), coagulation studies, or hyponatremia are some of the more common that spring to mind. However, arguably the most foreboding is hypercalcemia that is directly linked to a malignancy.
Hypercalcaemia of malignancy is a relatively common manifestation of cancer, occurring in between 10-30% of all cases of solid or haematological malignancies. It rarely presents at initial diagnosis (1-5% according to one study [1]), but the incidence increases as the disease becomes more advanced and heavily pretreated.
Hypercalcemia is also associated with an incredibly poor prognosis: one study from 1990 [2] reported a median survival of 30 days in a subset of patients who did not receive anticancer therapy. While the outlook for patients has improved with increasing awareness, it remains a very poor prognostic sign. The most common solid organ malignancies that cause hypercalcemia are breast cancer, renal cell cancer, lung cancer and squamous cell cancers of any origin.
The underlying mechanism of malignant hypercalcemia varies. The most common mechanisms include [3]:
Osteolytic metastases: causes hypercalcaemia due to excessive calcium release from bones. May be mediated by the local release of parathyroid hormone-related protein (PTHrP) from the release of RANK/RANK-ligand interaction, excessive osteoclast activity and bone resorption
Humoural: inappropriate bone resorption and hypercalcemia mediated by secretion of systemic PTHrP. According to one review [3], this mechanism is responsible for the vast majority (80%) of cases of hypercalcemia of malignancy and is associated with squamous cell carcinomas (head, lung and neck, esophageal, cervical, and colon cancers), breast, kidney, bladder, endometrial and ovarian cancers
Ectopic secretion of parathyroid hormone (PTH): accounts for <1% of cases, and may be part of a para-neoplastic syndrome secondary to lung or neuroendocrine cancers.
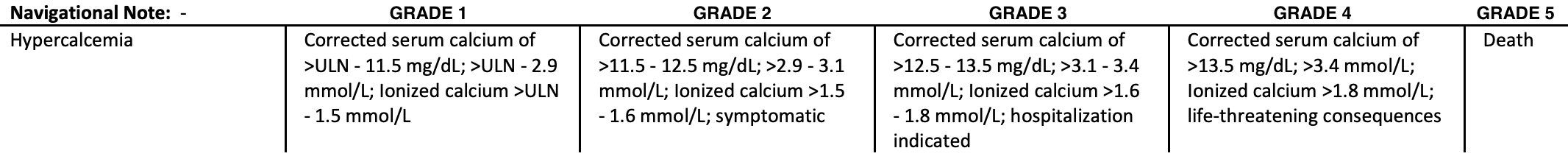
According to the Common Terminology Criteria for Adverse Events (CTCAE) v.5.0, hypercalcemia is defined as follows:
Courtesy of National Institute of Health (NIH) [4]
There are a couple of pitfalls of which it is important to be aware:
Cancer patients are frequently malnourished, which manifests biochemically as a low serum albumin. A normal or near-normal serum albumin is required to calculate a corrected calcium (cCa) value. As such, when a cCa is unavailable, ionised calcium from venous blood gas (VBG) should be used to gain accurate measurement.
The equation to manually calculate corrected calcium is as follows: [Corrected calcium (mmol/L) = serum calcium (mmol/L) + 0.02 (40 - serum albumin (g/L)) ] [5]
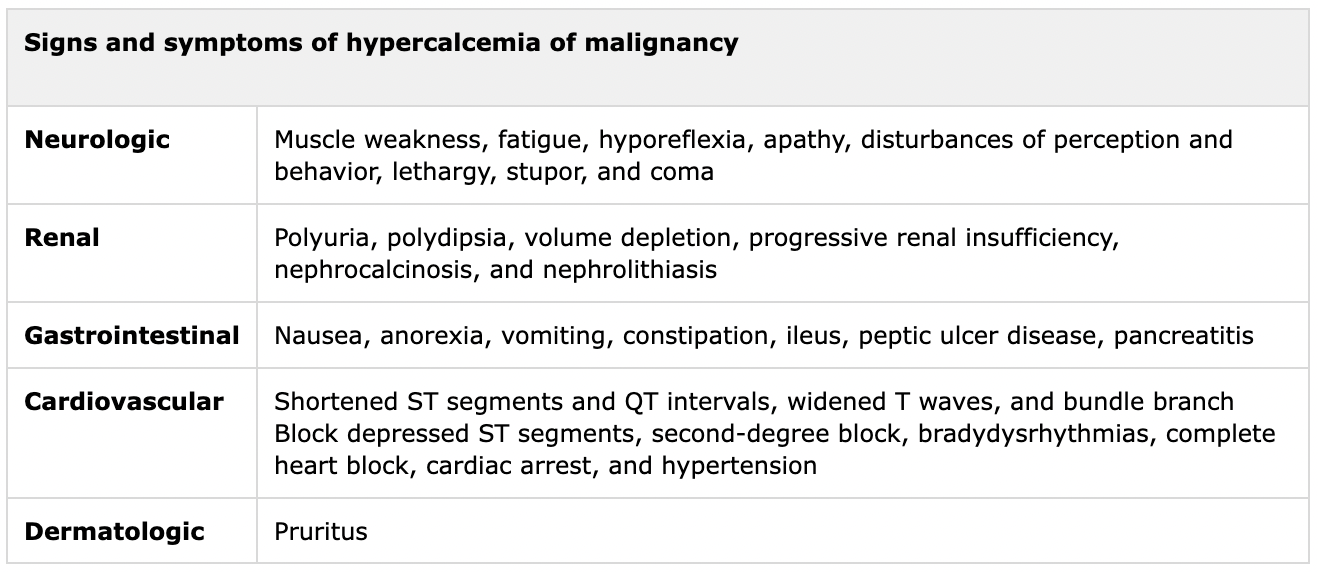
Clinical manifestations of hypercalcemia are determined by the absolute calcium value and the acuity of change; a patient with grade 2 or 3 hypercalcemia may be asymptomatic if the elevation is subacute or chronic [6].
Patients with severe hypercalcemia are almost universally symptomatic. Common clinical manifestations of hypercalcemia from malignancy are summarised below:
Table 1. Courtesy of the Journal or Hormone and Metabolic Research [3]
In addition to the measurement of serum calcium, other initial investigations for the patient with hypercalcemia of malignancy include:
Urea, electrolytes and creatinine
Serum magnesium and phosphate
Serum parathyroid hormone (PTH) to exclude primary hyperparathyroidism
PTHrP to rule out humoral hypercalcaemia of malignancy
1,25-dihydroxy vitamin D (1,25(OH)2D)
25-hydroxy vitamin D (25(OH)D) to exclude vitamin D intoxication
Thyroid-stimulating hormone (TSH)
Electrocardiogram (ECG)
In the acute presentation, the backbone of therapy is fluid resuscitation; in the setting of polyuria and reduced oral intake, the majority of patients present with dehydration. Increasing intravascular volume with isotonic saline increases renal perfusion and, thus, renal calcium excretion. In clinical practice, this is commonly combined with frusemide to increase secretion, but this addition is most commonly reserved for patients with cardiovascular comorbidities.
The addition of bisphosphonates (e.g., IV zoledronic acid) or denosumab is frequently used after fluid resuscitation and improvement of renal function. Zoledronic acid (ZA) has been associated with nephrotoxicity, and denosumab may cause “rebound” hypocalcemia in patients with renal impairment.
Calcitonin is a rapid-acting peptide hormone secreted by the thyroid gland that inhibits osteoclastic bone resorption and renal calcium reabsorption. It is used as an adjunct treatment for patients with severe hypercalcemia. In combination with bisphosphonates, it can rapidly lower calcium levels, but is associated with rapid tachyphylaxis within three days in the setting of calcitonin receptor downregulation.
However, the mainstay of treatment of hypercalcemia over the medium and long term is treating the underlying disease process. Systemic therapy should be commenced as soon as is clinically appropriate.
The Bottom Line:
Malignant hypercalcemia is a common and serious manifestation of cancer. It is associated with severe symptoms, advanced disease and a very poor prognosis. Investigations are focussed on ruling out an alternative aetiology and examining for renal and cardiovascular complications. Initial treatment is focussed on volume expansion, with the potential addition of bisphosphonates/denosumab and calcitonin.
Sources.
Stewart AF. Hypercalcemia Associated with Cancer. New England Journal of Medicine [Internet]. 2005;352(4):373–9. Available from: https://www.nejm.org/doi/full/10.1056/NEJMcp042806
Stuart H. Ralston, Stephen J. Gallacher, Uday Patel, et al. Cancer-Associated Hypercalcemia: Morbidity and Mortality: Clinical Experience in 126 Treated Patients. Ann Intern Med.1990;112:499-504. doi:10.7326/0003-4819-112-7-499
Asonitis Anna; Zafeiris Christos; Lambrou George I; Dontas Ismene; Kassi Eva NA. Diagnosis, Pathophysiology and Management of Hypercalcemia in Malignancy: A Review of the Literature. Hormone and Metabolic Research [Internet]. 2019;51(12):770–8. Available from: http://www.thieme-connect.com/products/ejournals/abstract/10.1055/a-1049-0647
Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Published November 2017. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50
Payne RB, Little AJ, Williams RB. Interpretation of serum calcium in patients with abnormal serum proteins. Br Med J 1973; 4:643.