A Better Prostate Screening Regimen? Early Results from ProScreen
Early detection and treatment of cancer is the best way to ensure good patient outcomes and prolonged survival. While targeted screening guidelines and recommendations, such as those for family members of patients with colorectal cancer, are important on an individual level, the Holy Grail of cancer prevention remains large, non-invasive screening programs. These best examples of such programs include regular faecal occult blood testing (FOBT) for colorectal cancer and the widespread use of screening mammograms and ultrasounds (MMG/USS) for breast cancer. Thanks to such programs, “screen-detected” disease is now fairly common and, in many cases, allows for curative — rather than palliative — treatment.
What defines a good screening program? In an ideal world, the test would have a high specificity (low false positive rate) and sensitivity (low false negative rate). However, in practice, tests often have to choose between sensitivity and specificity; of the two, sensitivity is more important, as a population-based program would rather over-diagnose than under-diagnose patients. The FOBT program, for example, has a higher sensitivity because blood in stool is rarely “normal”; however, the test specificity is low because there are many causes of a positive test, and most are not malignant.
There are also practical and logistical considerations. A screening test should be easy to scale up to a large population, meaning it needs to be cheap, simple and easy to interpret and escalate. Most screening programs take the form of blood or stool tests or widely available, inexpensive radiological investigations, such as MMG, USS and low-dose CTs for lung cancer. The results must be interpretable by general practitioners — a simple “yes” or “no” is ideal — without the need for specialist assistance for interpretation.
There also needs to be very clear protocols or guidelines regarding further investigation of abnormal results. A positive FOBT necessitates a referral to a gastroenterologist or surgeon for endoscopic investigation; a positive MMG/USS will lead to a referral to a breast surgeon for potential biopsy and resection.
These considerations lead us to prostate cancer. Prostate cancer remains the second most common malignancy and the fifth leading cause of cancer death among men worldwide. In the UK in 2019, more than 47,000 men were diagnosed with prostate cancer, while aggressive prostate cancer was responsible for more than 12,000 deaths in the same year. However, the use of prostate-specific antigen (PSA) in the screening space has been very controversial. 10-year results from the Cluster Randomized Trial of PSA Testing for Prostate Cancer (CAP) demonstrated no mortality benefit from a single-test PSA screen of a general population, compared to no screening at all (RR 0.96, p=0.50). Despite this, PSA remains the most practical screening tool available.
However, early results from the ProScreen Randomised Trial, published in JAMA by Auvinen et al. (April 6, 2024), have suggested that expanding the prostate cancer screening panel beyond PSA may improve not only the sensitivity of prostate screening, but also differentiate between patients with aggressive and low-grade prostate cancer.
Image courtesy of JAMA, 2024
The study made use of PSA, a 4-kallikrein panel and a multiparametric MRI to differentiate patients with and without prostate cancer, and differentiate between high and low-grade disease. The 4K test includes blood levels of total, free and intact PSA as well as human kallikrein-related peptide 2 (hK2). hK2 is one of a family of peptides primarily localised to the prostate and minimally secreted by non-prostate tissue. In addition, hK2 is more highly expressed in poorly-differentiated prostate cell than other kallikrein-related peptides.
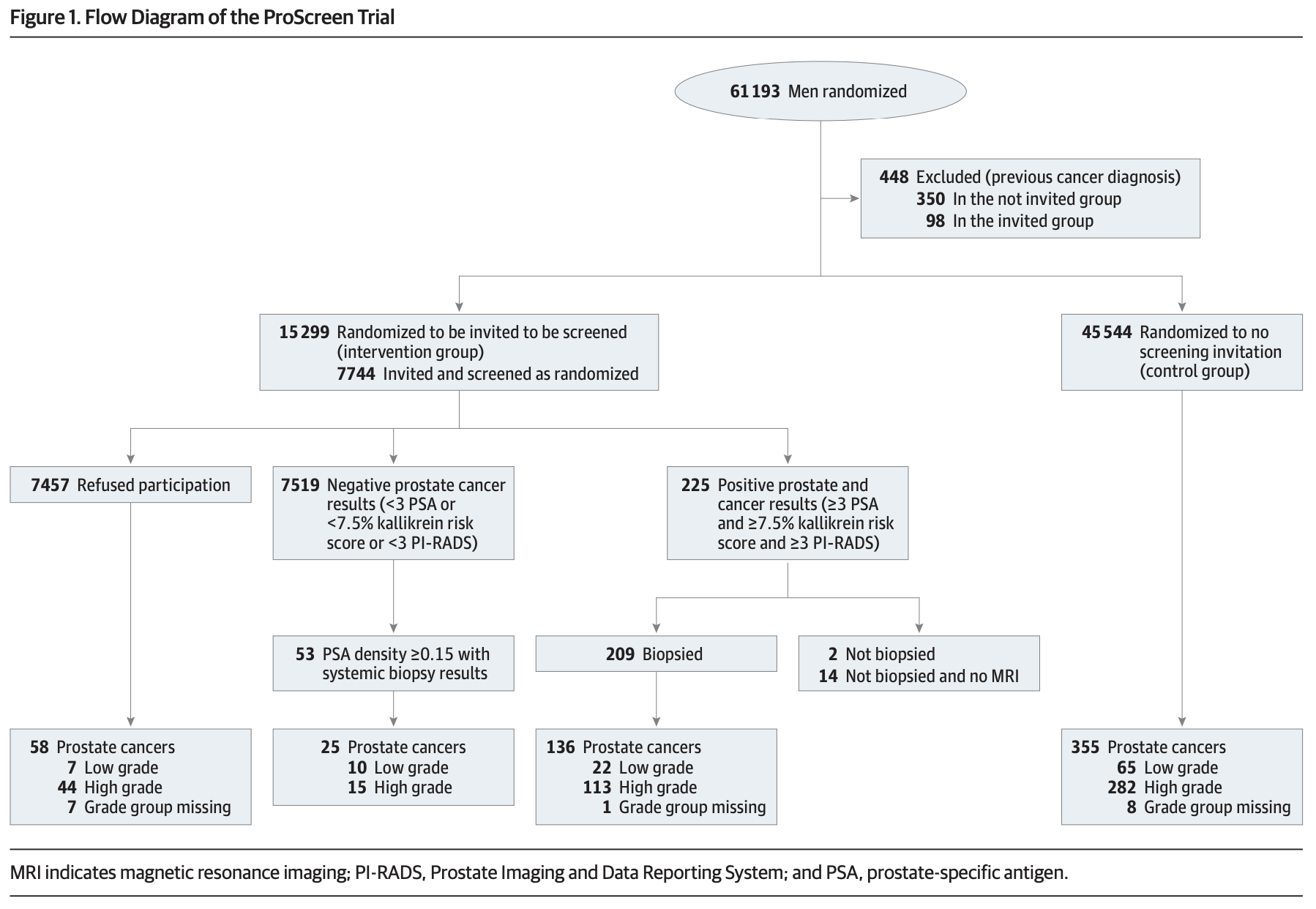
The study was conducted in Finland and enrolled patients between the ages of 50 and 63 to allow for at least two screening opportunities before age 71. Participants were randomised to the screening program or to the control group that did not receive any investigations. The screening program consisted of measurements of PSA, a 4K panel and a baseline multiparametric MRI for initial assessment. The re-screening interval was stratified according to the first-round PSA test results:
PSA 3.0ng/mL or higher: 2 years
PSA 1.5-2.99ng/mL: 4 years
PSA <1.5ng/mL: 6 years
Those who declined screening invitations were invited to subsequent screening rounds. Men with kallikrein panel risk scores of 7.5% or higher were referred to a local urology department for prostate MRI for Prostate Imaging Reporting and Data System (PI-RADS) analysis and potential biopsy.
Image courtesy of JAMA, 2024
The primary endpoint was prostate cancer mortality, with secondary outcomes including the incidence of high-grade prostate cancer (prostate cancer grade groups [GGs] 2-5) and low-grade (GG 1) cancer, the incidence of advanced disease (defined as T3-4, N1 or M1), frequency of biopsy referral, quality of life and cost-effectiveness.
60,745 patients were randomised: 15,201 to the screening group and 45,544 to the control group. The mean age at study entry was 57.3 years; interestingly, older patients were more likely to participate fully in the screening program (54.5% for 60-64 years, compared to 47.3% for 50-54 years).
Image courtesy of JAMA, 2024
526 men had PSA values of 3ng/mL or higher, and kallikrein panel scores of 7.5% or higher. 97% of these underwent MRI, of which 41% had a PI-RADS score of 3-5, suggestive of high-grade cancer. Transrectal prostate biopsies were performed in 209 (99%) of these high-risk patients (2.7% of total screening participants), 136 (65%) of which demonstrated prostate cancer. The number of biopsies needed to detect cancer in this group was 1.5. Of those cases, 113 were high-grade (52 GG2 and 61 GG3-5).
53 patients had a PI-RADS score <3 but a PSA density of 0.15ng/L squared or higher. These patients were referred for a systematic biopsy, and 25 biopsies demonstrated cancer (10 GG1, 11 GG2, 4 GG3-5). Detection of high-grade cancer increased with age, PSA value, the 4-kallikrein panel risk score.
For reference, among the 45,544 men in the control group 355 new prostate cancers were detected during a median follow-up of 3.2 years, of which 282 (79%) were high-grade histopathology.
So what does all this mean?
The first thing to note is that the study remains in progress, and drawing definitive conclusions on the program’s mortality benefit from available data is impossible. While the screening program appears to be more efficacious in detecting prostate cancer, that is unsurprising given the investigations involved.
In addition, the use of multiparametric MRI at patients’ baseline screening visits may prove to be a limiting factor to this program in many larger, more sparsely-populated countries or those with lower SES. In Australia, for example, the availability of MRIs varies significantly by geographic location. As a result, for a population-based screening program, the benefit may be limited.
However, despite these limitations, one number leaps from the page: 1.5. This is the number of biopsies required to detect cancer after completing the prescribed screening program. Ideally, the number needed to treat or detect (NNT) should be as close to 1 as possible; an NNT of 1 means that every test will detect cancer. Given the predominance of high-grade cancer in this group, it is possible that the screening program will lead to earlier detection, more patients undergoing — or at least be provided with the option of — definitive management and prolonged survival.
For now, however, the best that communities and general practitioners can do is judiciously use PSA for population screening, with a low threshold to refer patients with high-risk features for radiological or urological evaluation.
Source:
Auvinen A, Tammela TLJ, Mirtti T, et al. Prostate Cancer Screening With PSA, Kallikrein Panel, and MRI: The ProScreen Randomized Trial. JAMA. Published online April 06, 2024. doi:10.1001/jama.2024.3841
Other useful links:
Royal Australian College of General Practitioners guidelines for colorectal screening. Available from: https://www1.racgp.org.au/ajgp/2018/december/colorectal-cancer-screening-in-australia
Martin RM, Donovan JL, Turner EL, et al. Effect of a Low-Intensity PSA-Based Screening Intervention on Prostate Cancer Mortality: The CAP Randomized Clinical Trial. JAMA. 2018;319(9):883–895. doi:10.1001/jama.2018.0154
Rittenhouse, H. G., Finlay, J. A., Mikolajczyk, S. D., & Partin, A. W. (1998). Human Kallikrein 2 (hK2) and Prostate-Specific Antigen (PSA): Two Closely Related, but Distinct, Kallikreins in the Prostate. Critical Reviews in Clinical Laboratory Sciences, 35(4), 275–368. https://doi.org/10.1080/10408369891234219